Mass Spectrometer
Mass spectrometry can be used to identify compounds
because different compounds all produce a unique pattern of relative abundances
of isotopes. It was used by the Viking space probe, which landed on Mars to
identify elements on the Martian surface. A mass spectrometer can also be used
to calculate the relative atomic/isotopic mass of atoms.
Main stages of a mass spectrometer:
Vaporised
The sample is vaporised by using a high
temperature. A low pressure is also necessary to create a vacuum, which ensures
only the sample you want to test is present.
Ionisation
The
sample is bombarded by high energy electrons from an electron gun to produce
positive ions.
Acceleration
An
electric field produces negatively charged plates, which accelerates the ions.
Deflection
An
electromagnet creates a magnetic field, which defects the ions, with the degree
of deflection dependent on the mass/charge ratio (the lower the mass or the
greater the charge the greater the degree of deflection).
Detection
Arriving
ions produce an electric current that produces a mass spectrum, the greater
the area under the peak, the greater the abundance.
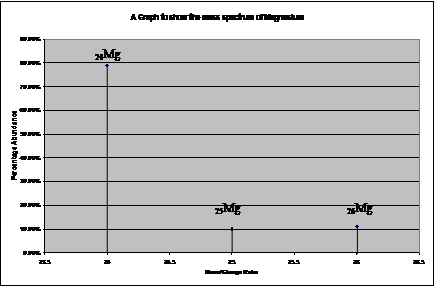
Mass Spectrum:
Example: The Relative isotopic mass and percentage
abundance of Magnesium:
|
Relative isotopic mass |
Percentage abundance |
| 24 |
78.99% |
| 25 |
10.00% |
| 26 |
11.01% |