Electronic Configuration
Electrons
are arranged in energy levels or shells, these are displayed as 2,8…
e.g. 13Al (2,8,3).
The
maximum number of electron in each shell are as follows:
|
Energy level |
Maximum number of electrons |
| n = 1 |
2 |
| n = 2 |
8 |
| n = 3 |
18 |
| n = 4 |
32 |
n
is referred to as the principal quantum number or shell number.
Sub-shells
Shells
can also be divided up into the sub-shells s, p, d and f.
|
Energy level |
Sub-shell |
Maximum number of electrons |
| n = 1 |
1s |
2 |
| n = 2 |
2s, 2p |
8 |
| n = 3 |
3s, 3p, 3d |
18 |
| n = 4 |
4s, 4p, 4d, 4f |
32 |
The
s sub-shell holds 2 electrons
The
p sub-shell holds 6 electrons
The
d sub-shell holds 10 electrons
The
f sub-shell holds 14 electrons
So
instead of writing (2…) in shells you can write it in sub-shells =
1s2…
Where 2 is the number of electrons
Where 1 is the principal quantum number
Where s is the name of the sub-shell
Note the 4th sub-shell
is lower in energy than the 3rd sub-shell so this begins to fill
before 3d.
Electron configuration in sub-shells: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 …
This pattern can be observed by moving across the
periodic table where each letter corresponds to a block.
Example
of the electronic configuration of Oxygen: 1s2 2s2 2p6
3s2 3p4
(Total
number of electrons 2 + 2 + 6 + 2 + 4 = 16)
Relationship between sub-shells and elements position in the periodic table
Sodium’s
electronic configuration using sub-shells is: 1s2 2s2
2p6 3s1
It
has 3 principal quantum shells (n=3) and therefore is in period three. It
is in s block and its outer electron is in the s sub-shell, it also has one
electron in its outer shell and so it is in group 1.
Orbital
This
is the volume of space around the atom in which there is good chance of finding
an electron.
Sub-shells
are made up of orbitals. Each orbital can only hold 2 electrons but more than
one orbital can form a sub-shell.
- For sub-shell s there
is only one orbital also known as s
- For sub-shell p there
are three orbitals known as px, py and pz
because there are three orbitals the p sub-shell can hold a maximum of 6
electrons
- For the d sub-shell there
are five orbitals which can all also hold two electrons, so the d sub-shell
can hold a total of 10 electrons
Orbital shapes
S
orbital

P orbitals



Order of filling orbitals
Paired
electrons in the same orbital will repel each other and therefore electrons
in the same orbital will spin in opposite directions to prevent repulsion.
Electrons therefore enter orbitals without any electrons in them first.
When drawing electrons in orbitals arrows are used to represent the direction the electrons are spinning. Therefore in each orbital there will be one arrow pointing downwards and the other upwards.
Electronic configuration of oxygen using orbitals:
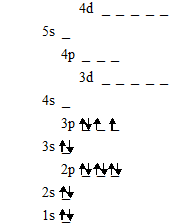
Note how the electrons occupy the empty orbitals first and then when these are all taken begin to pair up
Definition: Isoelectronic
Different
elements, which have the same electronic configuration e.g. Na and Mg+
both have 11 electrons, thus their electronic configuration is the same.
